Mechanistic PK-PD model of alendronate treatment of postmenopausal osteoporosis predicts bone site-specific response
Encoded in CellML by Julie Kim | Auckland Bioengineering Institute, University of Auckland
About this model
Original publication:
Calvo-Gallego, J. L., Pivonka, P., Ruiz-Lozano, R., & Martínez-Reina, J. (2022). Mechanistic PK-PD model of alendronate treatment of postmenopausal osteoporosis predicts bone site-specific response. Frontiers in bioengineering and biotechnology, 10, 940620.
DOI:
https://doi.org/10.3389/fbioe.2022.940620
Model status
-
This CellML model has been adapted from the author’s MATLAB code (See Supplementary material from the original publication for the MATLAB code).
-
The current CellML implementation focuses on the bone remodelling process and neglects the pharmacokinetics (PK) and pharmacodynamics (PD) of alendronate and microstructural damage.
-
The model runs in OpenCOR.
-
CellML Validation warns of unit inconsistency within this model due to usages of non-constant exponents, but the units are consistent throughout.
Model Structure
Abstract:
Alendronate is the most widely used drug for postmenopausal osteoporosis (PMO). It inhibits bone resorption, affecting osteoclasts. Pharmacokinetics (PK) and pharmacodynamics (PD) of alendronate have been widely studied, but few mathematical models exist to simulate its effect. In this work, we have developed a PK model for alendronate, valid for short- and long-term treatments, and a mechanistic PK-PD model for the treatment of PMO to predict bone density gain (BDG) at the hip and lumbar spine. According to our results, at least three compartments are required in the PK model to predict the effect of alendronate in both the short and long terms. Clinical data of a 2-year treatment of alendronate, reproduced by our PK-PD model, demonstrate that bone response is site specific (hip: 7% BDG, lumbar spine: 4% BDG). We identified that this BDG is mainly due to an increase in tissue mineralization and a decrease in porosity. The difference in BDG between sites is linked to the different loading and dependence of the released alendronate on the bone-specific surface and porosity. Osteoclast population diminishes quickly within the first month of alendronate treatment. Osteoblast population lags behind but also falls due to coupling of resorption and formation. Two dosing regimens were studied (70 mg weekly and 10 mg daily), and both showed very similar BDG evolution, indicating that alendronate accumulates quickly in bone and saturates. The proposed PK-PD model could provide a valuable tool to analyze the effect of alendronate and to design patient-specific treatments, including drug combinations.
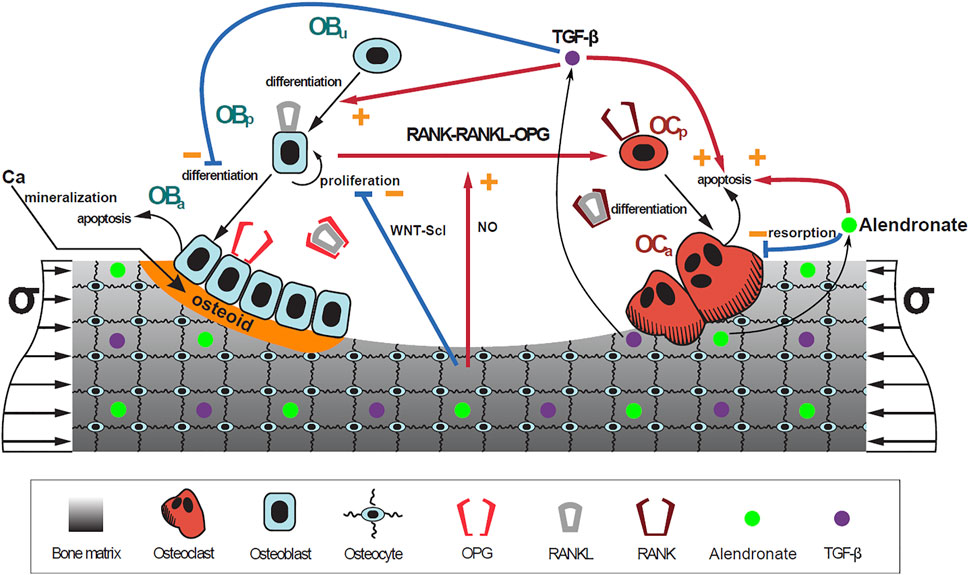
|
| A schematic representation of the model: bone cell differentiation stages along with biochemical and biomechanical interactions are presented. The mineralization of osteoid is shown in orange (Figure 3). Note: alendronate is not modelled in the current CellML implementation. |

