De Vries, Sherman, 2001
Model Status
This model integrates in OpenCell to produce an oscillating output but this output has not been checked against the publication.
ValidateCellML confirms this model as valid CellML but detects unit inconsistencies. We have checked this model and the unit inconsistency appears to be inherent in the publication. Attempting to balance the units by changing the units of tau from milliseconds to picofarads results in a broken simulation output.
Model Structure
When exposed to a threshold concentration of glucose, pancreatic beta-cells exhibit a complicated pattern of electrical activity. Bursts of action potential spikes (the "active" phase) are observed, separated by a "silent" phase of membrane repolarisation. At even higher glucose concentrations, continuous action potentials are seen. This electrical activity has two important physiological correlates: increased cytosolic Ca2+ concentration ([Ca2+]i) and increased rate of insulin secretion during the active phase. It is generally accepted that the rise in [Ca2+]i plays a major role in insulin secretion and that the action potential spikes during a burst are responsible for the rise in [Ca2+]i.
Normal bursting patterns are only observed when the beta-cells act synchronously, as they do in vivo, in electrically coupled organs called the islets of Langerhans. Isolated spiking cells, incapable of bursting by themselves, may generate islet-like bursting rhythms when coupled. There are three alternative hypotheses to explain why single cells and islets are different. One of these emphasises heterogeneity: single cell properties are variable, and individual cells may fall outside the narrow parameter regime required for bursting. By contrast, in coupled populations, cells with an excess of current can be balanced by cells with too little, and the population is more likely to achieve bursting. A second hypothesis focuses on noise from stochastic channel fluctuations in individual cells preventing bursting by causing premature transitions between active and silent phases. However, in large populations, fluctuations are buffered, allowing the underlying burst dynamics to be dominant. A third alternative discusses the paracrine effects of glucagon secreted by alpha-cells in the islet, on beta-cells.
In their 2001 paper, Gerda De Vries and Arthur Sherman contrast the first two hypotheses. They introduce a mathematical model for bursting in pancreatic beta-cells, which is a simplified version of the biophysically based, earlier model by Sherman and Rinzel (1992). This model describes three ionic currents (see the figure below): a fast voltage-activated calcium current, ICa; a delayed rectifier potassium current, IK; and a very slow inhibitory potassium current, Is. The calcium and delayed rectifier potassium currents are responsible for generating action potentials. The slow potassium current plays no essential role in the individual cells but heterogeneity among the cells is generated by varying the value of the parameter beta. Parameter values were chosen such that the individual cells are incapable of bursting. De Vries and Sherman use their model to show bifurcation in a two-cell system, and they extrapolate this for larger clusters of coupled cells.
The complete original paper reference is cited below:
From Spikers to Bursters Via Coupling: Help From Heterogeneity, Gerda De Vries and Arthur Sherman, 2001, Bulletin of Mathematical Biology , 63, 371-391. PubMed ID: 11276531
The raw CellML description of the model can be downloaded in various formats as described in
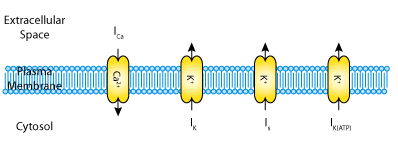 |
| A schematic representation of the three transmembrane currents captured by the De Vries and Sherman 2001 pancreatic beta-cell model. |

