Potter, Greller, Cho, Nuttall, Stroup, Suva, Tobin, 2005
Model Status
This CellML version of the model has been checked in COR and OpenCell. The units have been checked and are correct and are consistent. The model runs in COR and Opencell to it recreate the published results. This model recreates continuous PTH dosing model using low doses.
Model Structure
ABSTRACT: In this paper, we propose a mathematical model for parathyroid hormone receptor (PTH1R) kinetics, focusing on the receptor's response to PTH dosing to discern bone formation responses from bone resorption. The PTH1R is a major target for new osteoporosis treatments, as pulsatile PTH dosing has been shown to induce net bone formation in both animals and humans, and PTH(1-34) was recently FDA approved for the treatment of post-menopausal osteoporosis. PTH has also been shown to cause net bone loss when given continuously, so that the net action of PTH on bone is dependent on the dosing pattern. We have developed a simplified two-state receptor kinetics model for the PTH1R, based on the concepts of Segel et al., to distinguish the activity of active and inactive receptor and receptor-ligand complexes. The goal is to develop a plausible model of the minimal essential biological relationships necessary for understanding the responses to PTH dosing. A two-state model is able to effectively discriminate between continuous and pulsatile PTH dosing using the active species as surrogates for the downstream anabolic response. For continuous PTH dosing, the model predicts a desensitized system dominated by the inactive receptor and complex, consistent with downstream net bone loss that has been demonstrated experimentally. Using pulsatile PTH dosing, the model system predicts a highly sensitized state dominated by the active receptor and complex, corresponding to net bone formation. These results are consistent with the hypothesis that the kinetics of the receptor plays a critical role in the downstream effects of PTH dosing. Moreover, these results indicate that within a range of biologically relevant PTH doses, the two-state model is able to capture the differential behavior of the system for both continuous and pulsatile PTH dosing. The development of such a model provides a rational basis for developing more biologically extensive models that may support the design of optimal dosing strategies for PTH-based anti-osteoporosis treatments. Moreover, this model provides a unique starting point from which to design experiments investigating PTH receptor biology.
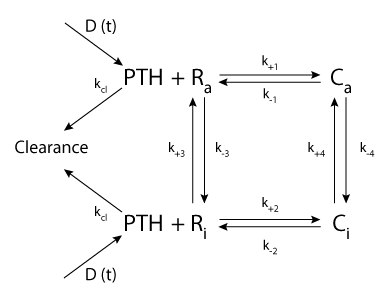 |
| Schematic diagram of the two-state model for PTHR1 binding kinetics PTH is secreted and/or dosed at a rate D and binds to the active (Ra) and inactive (Ri) forms of the receptor. PTH-PTHR1 binding results in the formation of the active (Ca) and inactive (Ci) complexes. In addition, there is a conversion between the active and inactive forms of the receptor and complexes, while unbound PTH is cleared from the system. |
The original paper reference is cited below:
Response to continuous and pulsatile PTH dosing: a mathematical model for parathyroid hormone receptor kinetics, Laura K. Potter, Larry D. Greller, Carolyn R. Cho, Mark E. Nuttall, George B. Stroup, Larry J. Suva, and Frank L. Tobin, 2005, Bone, 37, 159-169. PubMed ID: 15921971

