Novak, Csikasz-Nagy, Gyorffy, Chen, Tyson, 1998
Model Status
This CellML version of the model runs in COR and PCEnv. Units have been checked and are consistent. In order to reproduce the figures in the published paper, initial concentrations for the variables need to be obtained from the author, as they are not listed in the paper.
Model Structure
During mitotic cell division, eukaryotic cells will replicate their DNA during the S-phase of the cell cycle, and then divide during the M-phase (see the figure below). S and M phases are temporally separated by gaps, G1 and G2 phases. These discrete phases of the cell cycle are carefully regulated by specific proteins, and in most eukaryotic cells, cell cycle events are coordinated at three checkpoints:
-
In G1 phase, cells analyse both their external environment and their intracellular state before committing to a new cycle of DNA synthesis and division.
-
In the G2 phase, the cell checks that DNA synthesis and repair are complete, and that the cell has reached a critical size before mitosis is initiated.
-
Thirdly, at metaphase, the cell ensures that all chromosomes are correctly aligned on the central plate before they are separated to opposite poles of the spindle.
Over the past few years advances in molecular genetics have lead to the elucidation of many of the molecular details of these control mechanisms. But although cyclin-dependent protein kinases (CDKs) are known to trigger the major events of the cell cycle, the precise mechanisms of the checkpoint controls are still uncertain.
Concurrent with advances in experimental techniques and the generation of molecular data, mathematical models of increasing complexity have evolved. In the study described here, Novak et al. develop a mathematical model of the fission yeast cell cycle with checkpoint controls at the G1/S, G2/M and metaphase/anaphase transitions (see the figure below). Much is known about the physiology, genetics and molecular biology of the fission yeast (Schizosaccharomyces pombe). By varying the parameter values of their model, the authors were able to represent both wild-type and wee1- mutant cells. They found that their model was consistent with the observed phenotypes of many different mutant fission yeast strains, and it also was able to predict interesting phenotypes for some multiple-mutant strains that have not yet been constructed and analysed.
The complete original paper reference is cited below:
Mathematical model of the fission yeast cell cycle with checkpoint controls at the G1/S, G2/M and metaphase/anaphase transitions, Bela Novak, Attila Csikasz-Nagy, Bela Gyorffy, Kathy Chen, and John J. Tyson, 1998, Biophysical Chemistry , 72, 185-200. (A PDF version of the article is available to subscribers on the Biophysical Chemistry website.) PubMed ID: 9652094
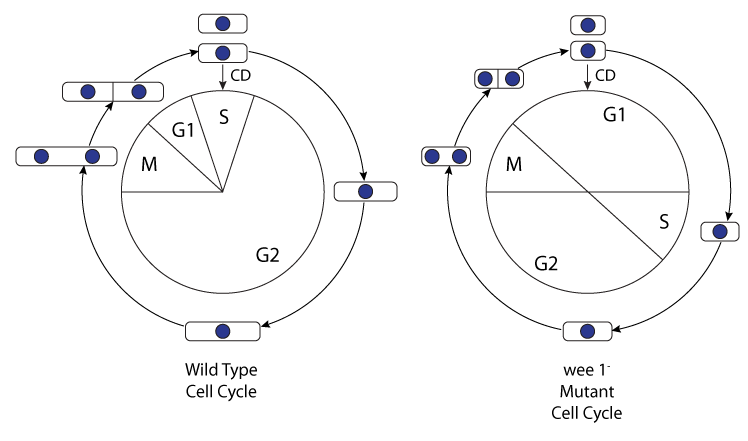 |
| The fission yeast cell cycle. The wild-type cell cycle and the wee1- mutant cell cycle are compared. The relative durations of the different phases differ, and also, the size of the cells at division differ, with wild-type cells being 14 micrometres in length, and wee1- cells bing just 8 micrometres in length. |
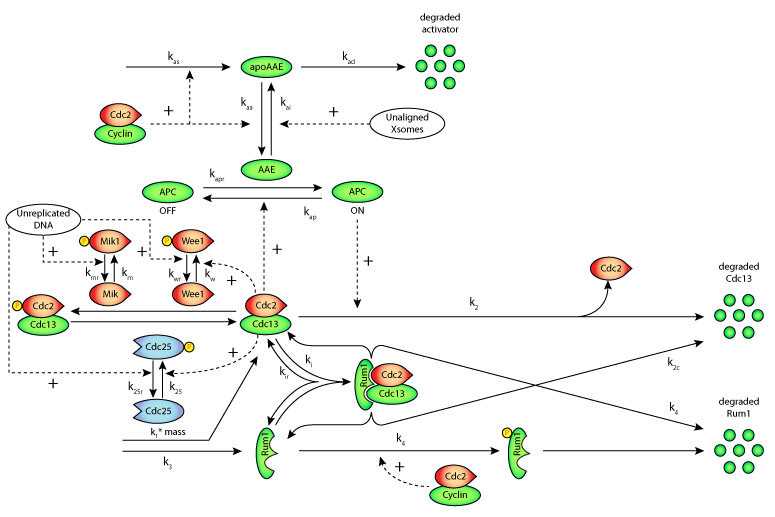 |
| Schematic diagram of a proposed molecular mechanism for three checkpoint controls in the fission yeast cell cycle. The G1/S transition is controlled by CDK-Rum1 interactions; the G2/M transition by Wee1-Mik1-Cdc25-controlled tyrosine-phosphorylation of CDK; and the metaphase/anaphase transition is regulated by an APC activating enzyme (AAE). |

