Dumaine, Towbin, Brugada, Vatta, Nesterenko, Nesterenko, Brugada, Brugada, Antzelevitch, 1999
Model Status
This is the original unchecked version of the model imported from the previous CellML model repository, 24-Jan-2006.
Model Structure
Of the 300,000 sudden deaths that occur in America each year, 5 to 12 percent are due to polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF) developing in patients with structurally normal hearts. About half of these cases are attributed to the Brugada syndrome, which is characterised by an ST-segment elevation in V1 through V3 and a rapid VT that can degenerate into VF. The disease has been linked to mutations in the SCN5A gene, which encodes the alpha subunit of the cardiac sodium channel. This temperature dependent mutation appears to change the gating kinetics of the sodium channel, such that the net outward current is augmented during the early phases of the right ventricular action potential.
In their 1999 study, Dumaine et al. tested this temperature dependence hypothesis. They studied cardiac action potentials by using a modified version of the Luo-Rudy II model. The modification involved adding a transient outward potassium current (Ito ) to the original model, and reducing the conductance of the L-type calcium channel by 20 to 50 percent. For a diagram of the model, please see the figure below.
The results of their study showed that this mutation is only expressed at physiological temperatures. This explained why the mutation phenotype had not been observed in previous experiments which had been carried out at room temperature. Their findings also suggest that some patients may be more at risk during febrile states when their body temperature is elevated.
The complete original paper reference is cited below:
Ionic Mechanisms Responsible for the Electrocardiographic Phenotype of the Brugada Syndrome Are Temperature Dependent, Robert Dumaine, Jeffrey A. Towbin, Pedro Brugada, Matteo Vatta, Dmitri V. Nesterenko, Vladislav V. Nesterenko, Josep Brugada, Ramon Brugada, and Charles Antzelevitch, 1999, Circulation Research , 85, 803-809. (Full text and PDF versions of this article are available to subscribers on the Circulation Research website). PubMed ID: 10532948
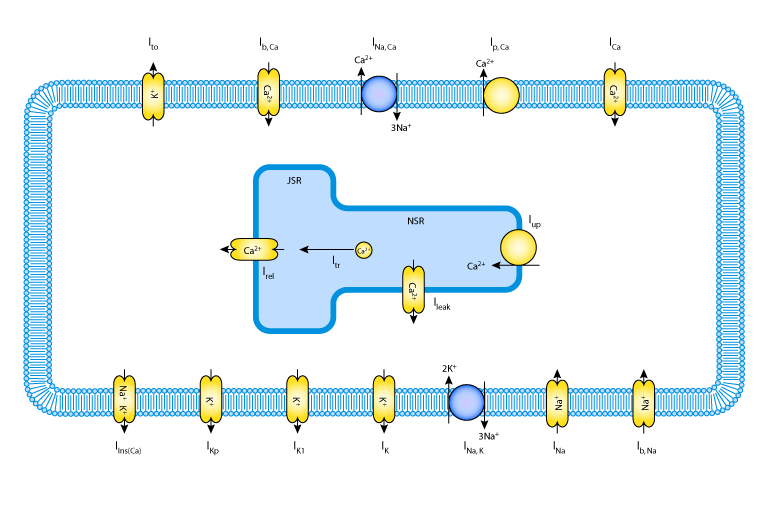 |
| A schematic diagram describing the ionic currents, pumps and exchangers that are captured in the Dumaine et al. 1999 model. The intracellular compartment is the sarcoplasmic reticulum (SR), which is divided into the two subcompartments, the network SR (NSR) and the junctional SR (JSR). Ca2+ buffers are present in both the cytoplasm and the JSR. |

